Synthesis of precious metals
Synthesis of precious metals refers to the realization of the age-old dream of alchemists: to artificially produce precious metals. This synthesis is only possible with methods utilizing nuclear physics, currently involving either nuclear reactors or particle accelerators.
Since particle accelerators require huge amounts of energy while nuclear reactors produce energy, production methods using a nuclear reactor are considered more economically feasible. Often the goal of synthesis is to produce an element at a cost significantly less than the standard methods of production. Recovery of rare elements from spent fuel rods is also anticipated to help offset the cost of reprocessing.
Contents |
Rhodium, Ruthenium
Rhodium and ruthenium are precious metals produced by nuclear fission, as a small percentage of the fission products. The radio-isotopes of these elements with the longest half-life, which are generated by nuclear fission have half-life times of 45 days and 373.59 days for rhodium and ruthenium, respectively. This makes their extraction from spent nuclear fuel possible, although they must be checked for radioactivity before use.[1]
Until now no facility has been reprocessing spent nuclear fuels for rhodium and ruthenium; however, Japan is planning to do so in their new spent fuel reprocessing facility, which will help offset the cost of reprocessing.
Ruthenium
Each kilogram of the fission products of 235U will contain 63.44 grams of ruthenium isotopes with halflives longer than a day. Since a typical used nuclear fuel contains about 3% fission products, one ton of used fuel will contain about 1.9 kg of ruthenium. The 103Ru and 106Ru will render the fission ruthenium very radioactive. If the fission occurs in an instant then the ruthenium thus formed will have an activity due to 103Ru of 109 TBq g−1 and 106Ru of 1.52 TBq g−1. Ru 103 has a half-life of about 39 days meaning that within 390 days it will have effectively decayed to ground state, well before any reprocessing is likely to occur. Ru 106 has a half-life of about 373 days meaning that if the fuel is left to cool for 5 years before reprocessing only about 3% of the original quantity will remain; the rest will have decayed to ground state.[1]
Another way to produce ruthenium would be to start with molybdenum, which has a price averaging between $10 and $20/kg, in contrast with ruthenium's $5500/kg.[2] The isotope 100Mo, which has an abundance of 9.6% in natural molybdenum, can be transmuted to 101Mo by slow neutron irradiation. 101Mo and its daughter product, 101Tc, both have beta-decay half-lives of roughly 14 minutes. The end product is stable 101Ru.
Rhodium
It is also possible to extract rhodium from used nuclear fuel, which contains rhodium (1 kg of the fission products of 235U contain 13.3 grams of 103Rh.) So as a typical used fuel is 3% fission products by weight it will contain about 400 grams of rhodium per ton of used fuel. The longest lived radioisotope of rhodium is 102mRh which has a half-life of 2.9 years, while the ground state (102Rh) has a half-life of 207 days.[1]
Each kilo of fission rhodium will contain 6.62 ng of 102Rh and 3.68 ng of 102mRh. As 102Rh decays by beta decay to either 102Ru (80%) (some Positron emission will occur) or 102Pd (20%) (some gamma ray photons with about 500 keV are generated) and the excited state decays by beta decay (electron capture) to 102Ru (some gamma ray photons with about 1 MeV are generated). If the fission occurs in an instant then 13.3 grams of rhodium will contain 67.1 MBq (1.81 mCi) of 102Rh and 10.8 MBq (291 μCi) of 102mRh. As it is normal to allow used nuclear fuel to stand for about five years before reprocessing, much of this activity will decay away leaving 4.7 MBq of 102Rh and 5.0 MBq of 102mRh. If the rhodium metal was then left for 20 years after fission then the 13.3 grams of rhodium metal would contain 1.3 kBq of 102Rh and 500 kBq of 102mRh. At first glance the rhodium might be adding to the resource value of reprocessed fission waste, but the cost of the separation of the rhodium from the other metals needs to be considered.[1]
Palladium
Palladium is also produced by nuclear fission in small percentages, amounting to 1 kg per ton of spent fuel. As opposed to rhodium and ruthenium, palladium has a radioactive isotope, 107Pd, with a very long half-life time of 6.5 million years, so palladium produced in this way has a very low radioactive intensity. Mixed in with the other isotopes of palladium recovered from the spent fuel, this gives a radioactive dose rate of 7.207x10−5 Ci, which is well below the safe level of 1x10−3 Ci. Also, 107Pd has a very low decay energy of only 33 KeV, and so would be unlikely to pose a hazard even if pure.
Silver
Silver is produced as result of nuclear fission in small amounts (approximately 0.1 %). Because of this, extraction of silver from highly radioactive fission products would be uneconomical, but when recovered with palladium, rhodium and ruthenium (price of silver in 2011: about 880 €/kg; rhodium; and ruthenium: about 300,000 €/kg) the economics change substantially. Silver becomes a byproduct of platinoid metal recovery from fission waste.
Gold
The artificial production of gold is the age-old dream of the alchemists. It is possible in particle accelerators or nuclear reactors, although the production cost is currently many times the market price of gold. Since there is only one stable gold isotope, 197Au, nuclear reactions must create this isotope in order to produce usable gold.
Gold synthesis from mercury
Gold obtained by mining has copper and silver impurities. Gold of higher purity can be made through the photoneutron process:
- Mercury 198 + 6.8MeV gamma ray
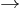 1 neutron + Mercury 197 (half-life 2.7 days
1 neutron + Mercury 197 (half-life 2.7 days 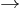 Gold 197 + 1 positron)
Gold 197 + 1 positron)
These energy levels allow a more efficient neutron source than the Spallation Neutron Source.
Gold synthesis in an accelerator
Gold synthesis in a particle accelerator is possible in many ways. The Spallation Neutron Source has a liquid mercury target that will be transmuted into gold, platinum, and iridium, which are lower in atomic number.
Gold synthesis in a nuclear reactor
Gold was first synthesized from mercury by neutron bombardment in 1941, but the isotopes of gold produced were all radioactive.[3]
Gold can currently be manufactured in a nuclear reactor by irradiation either of platinum or mercury.
Only the mercury isotope 196Hg, which occurs with a frequency of 0.15% in natural mercury, can be converted to gold by neutron capture, and following electron capture-decay into 197Au with slow neutrons. Other mercury isotopes are converted when irradiated with slow neutrons into one another or formed mercury isotopes, which beta decay into thallium.
Using fast neutrons, the mercury isotope 198Hg, which composes 9.97% of natural mercury, can be converted by splitting off a neutron and becoming 197Hg, which then disintegrates to stable gold. This reaction, however, possesses a smaller activation cross-section and is feasible only with un-moderated reactors.
It is also possible to eject several neutrons with very high energy into the other mercury isotopes in order to form 197Hg. However such high-energy neutrons can be produced only by particle accelerators.
Platinum
The cost of platinum as of January 2010 was $49,995 per kilogram, making it the second most expensive precious metal after rhodium. Iridium, by contrast, has only about one-fourth the value of platinum ($13,117 kg). Iridium has two naturally occurring isotopes, 191Ir and 193Ir. Irradiation by slow neutrons would transmute these isotopes into 192Ir and 194Ir, with short half-lives of 73 days and 19 hours, respectively; the predominant decay pathway for both of these isotopes is beta-minus decay into 192Pt and 194Pt.[4][5] Thus, iridium could be transmuted into more-valuable platinum.
Osmium
The cost of osmium as of January 2010 was $12,217 per kilogram, making it roughly twice the price of rhenium, which is worth $6,250/kg. Rhenium has two naturally occurring isotopes, 185Re and 187Re. Irradiation by slow neutrons would transmute these isotopes into 186Re and 188Re, which have half-lives of 3 days and 17 hours, respectively. The predominant decay pathway for both of these isotopes is beta-minus decay into 186Os and 188Os.[6][7] Thus, rhenium could be converted into more-valuable osmium.
Rhenium
The cost of rhenium as of January 2010 was $6,250/kg; by contrast, tungsten is very cheap, with a price of under $30/kg as of July 2010.[8] The isotopes 184W and 186W together make up roughly 59% of naturally-occurring tungsten. Slow-neutron irradiation could convert these isotopes into 185W and 187W, which have half-lives of 75 days and 24 hours, respectively, and always undergo beta decay to the corresponding rhenium isotopes.[9][10] The produced rhenium could then be transmuted to osmium (see above), increasing its value even further.
See also
References
- ^ a b c d Bush, R. P. (1991). "Recovery of Platinum Group Metals from High Level Radioactive Waste". Platinum Metals Review 35 (4): 202–208. http://www.platinummetalsreview.com/pdf/pmr-v35-i4-202-208.pdf.
- ^ "Molybdenum Price". http://www.infomine.com/investment/historicalcharts/showcharts.asp?c=molybdenum. Retrieved July 25, 2010.
- ^ R. Sherr, K. T. Bainbridge, and H. H. Anderson (1941). "Transmutation of Mercury by Fast Neutrons". Physical Review 60 (7): 473–479. Bibcode 1941PhRv...60..473S. doi:10.1103/PhysRev.60.473.
- ^ "Iridium 194". http://www.periodictable.com/Isotopes/077.194/index.dm.html.
- ^ "Iridium 192". http://www.periodictable.com/Isotopes/077.192/index.dm.html.
- ^ "Rhenium-186". http://www.periodictable.com/Isotopes/075.186/index.dm.html.
- ^ "Rhenium-188". http://www.periodictable.com/Isotopes/075.187/index.dm.html.
- ^ "Tungsten Prices". http://www.infomine.com/investment/historicalcharts/showcharts.asp?c=tungsten.
- ^ "Tungsten-185". http://www.periodictable.com/Isotopes/074.185/index.dm.html.
- ^ "Tungsten-187". http://www.periodictable.com/Isotopes/074.187/index.dm.html.
External links
- Spallation Neutron Source
- Mercury 197
- Mercury 197 decays to Gold 197
- "Potential Applications of Fission Platinoids in Industry". Platinum Metals Review 49 (2): 79. 2005. doi:10.1595/147106705X35263. http://www.platinummetalsreview.com/pdf/79-90-pmr-apr05.pdf.
- Kolarik, Zdenek; Renard, Edouard V. (2003). "Recovery of Value Fission Platinoids from Spent Nuclear Fuel. Part I PART I: General Considerations and Basic Chemistry". Platinum Metals Review 47 (2): 74–87. http://www.platinummetalsreview.com/pdf/pmr-v47-i2-074-087.pdf.
- Kolarik, Zdenek; Renard, Edouard V. (2003). "Recovery of Value Fission Platinoids from Spent Nuclear Fuel. Part II: Separation Process". Platinum Metals Review 47 (2): 123–131. http://www.platinummetalsreview.com/pdf/pmr-v47-i2-074-087.pdf.